Magdalena Leiva, Nuria Matesanz, Marta Pulgarín-Alfaro, Ivana Nikolić & Guadalupe Sabio.
The complex functions of adipose tissue have been a focus of research interest over the past twenty years. Adipose tissue is not only the main energy storage depot, but also one of the largest endocrine organs in the body and carries out crucial metabolic functions. Moreover, brown and beige adipose depots are major sites of energy expenditure through the activation of adaptive, non-shivering thermogenesis.
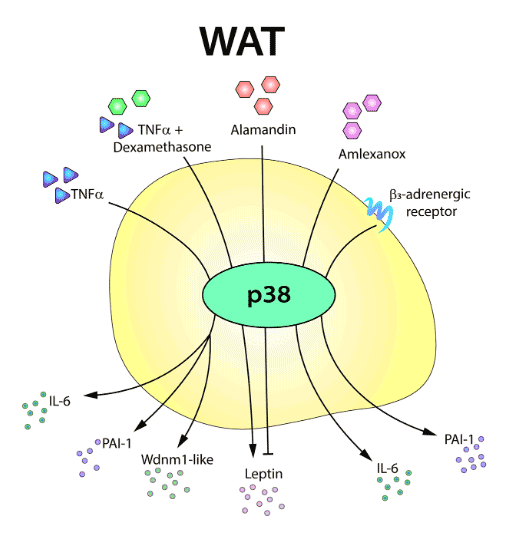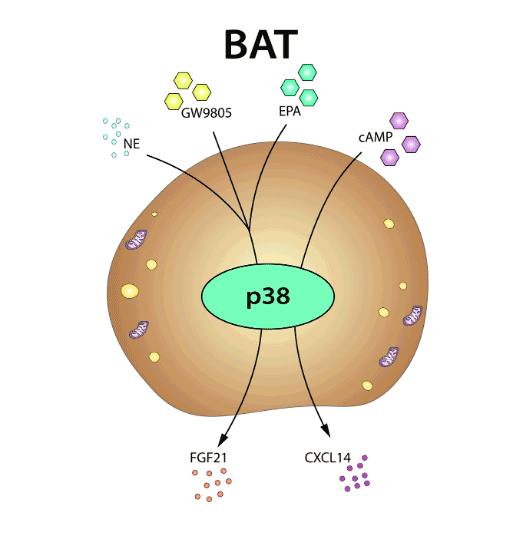
In recent years, numerous signaling molecules and pathways have emerged as critical regulators of adipose tissue, in both homeostasis and obesity-related disease. Among the best characterized are members of the p38 kinase family. The activity of these kinases has emerged as a key contributor to the biology of the white and brown adipose tissues, and their modulation could provide new therapeutic approaches against obesity.
Here, we give an overview of the roles of the distinct p38 family members in adipose tissue, focusing on their actions in adipogenesis, thermogenic activity, and secretory function.