Marcos F. Fondevila, Eva Novoa, Maria J. Gonzalez-Rellan, Uxia Fernandez, Violeta Heras, Begoña Porteiro, Tamara Parracho, Valentina Dorta, Cristina Riobello, Natalia da Silva Lima, Samuel Seoane, Maria Garcia-Vence, Maria P. Chantada-Vazquez, Susana B. Bravo, Ana Senra, Magdalena Leiva, Miguel Marcos, Guadalupe Sabio, Roman Perez-Fernandez, Carlos Dieguez, Vincent Prevot, Markus Schwaninger, Ashwin Woodhoo, Maria L. Martinez-Chantar, Robert Schwabe, Francisco J. Cubero, Marta Varela-Rey, Javier Crespo, Paula Iruzubieta, Ruben Nogueiras.
The p63 protein has pleiotropic functions and, in the liver, participates in the progression of nonalcoholic fatty liver disease (NAFLD). However, its functions in hepatic stellate cells (HSCs) have not yet been explored.
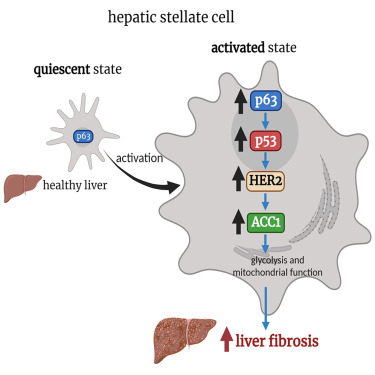
TAp63 is induced in HSCs from animal models and patients with liver fibrosis and its levels positively correlate with NAFLD activity score and fibrosis stage. In mice, genetic depletion of TAp63 in HSCs reduces the diet-induced liver fibrosis. In vitro silencing of p63 blunts TGF-β1-induced HSCs activation by reducing mitochondrial respiration and glycolysis, as well as decreasing acetyl CoA carboxylase 1 (ACC1).
Ectopic expression of TAp63 induces the activation of HSCs and increases the expression and activity of ACC1 by promoting the transcriptional activity of HER2. Genetic inhibition of both HER2 and ACC1 blunt TAp63-induced activation of HSCs. Thus, TAp63 induces HSC activation by stimulating the HER2-ACC1 axis and participates in the development of liver fibrosis.